Decoding the Realm of Acids and Bases: A Complete Information to the pH Scale and Past
Associated Articles: Decoding the Realm of Acids and Bases: A Complete Information to the pH Scale and Past
Introduction
With nice pleasure, we’ll discover the intriguing subject associated to Decoding the Realm of Acids and Bases: A Complete Information to the pH Scale and Past. Let’s weave fascinating info and supply contemporary views to the readers.
Desk of Content material
Decoding the Realm of Acids and Bases: A Complete Information to the pH Scale and Past
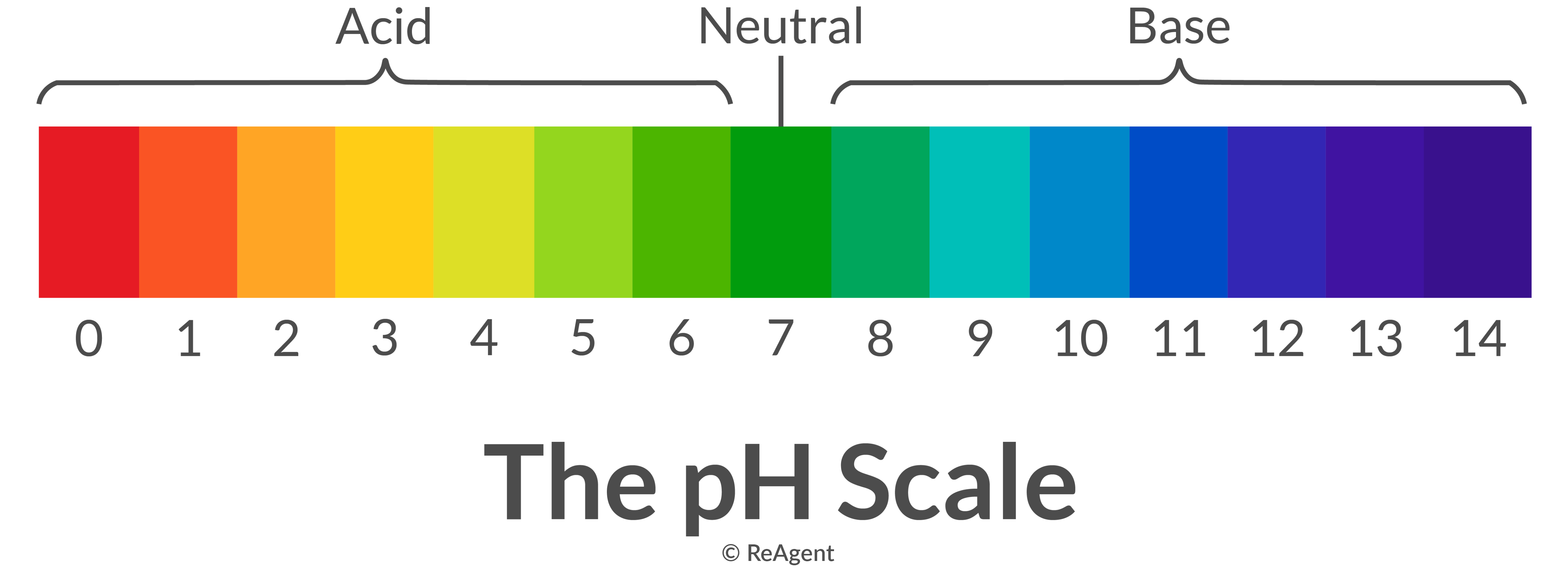
Acids and bases are elementary ideas in chemistry, impacting every thing from the processes inside our our bodies to industrial manufacturing. Understanding their properties and interactions is essential for a variety of scientific disciplines and on a regular basis purposes. This text delves deep into the world of acids and bases, offering a complete rationalization of the pH scale, completely different acid-base theories, and the sensible purposes of this information. We can even discover numerous strategies for figuring out and classifying acids and bases, culminating in an in depth dialogue of a sensible acid-base chart and its makes use of.
Understanding the pH Scale: A Measure of Acidity and Basicity
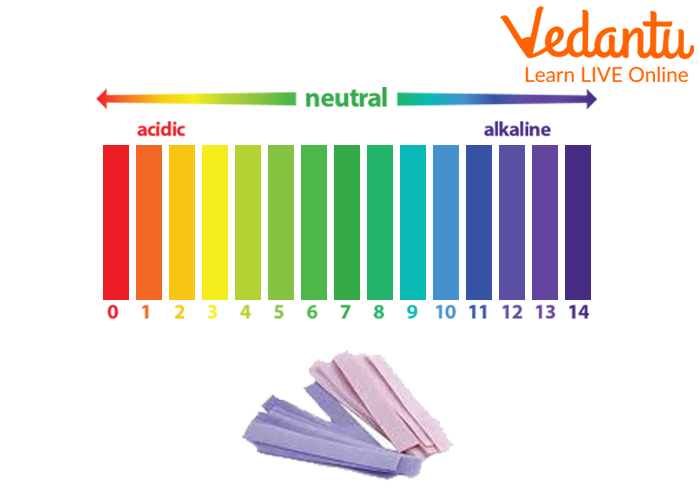
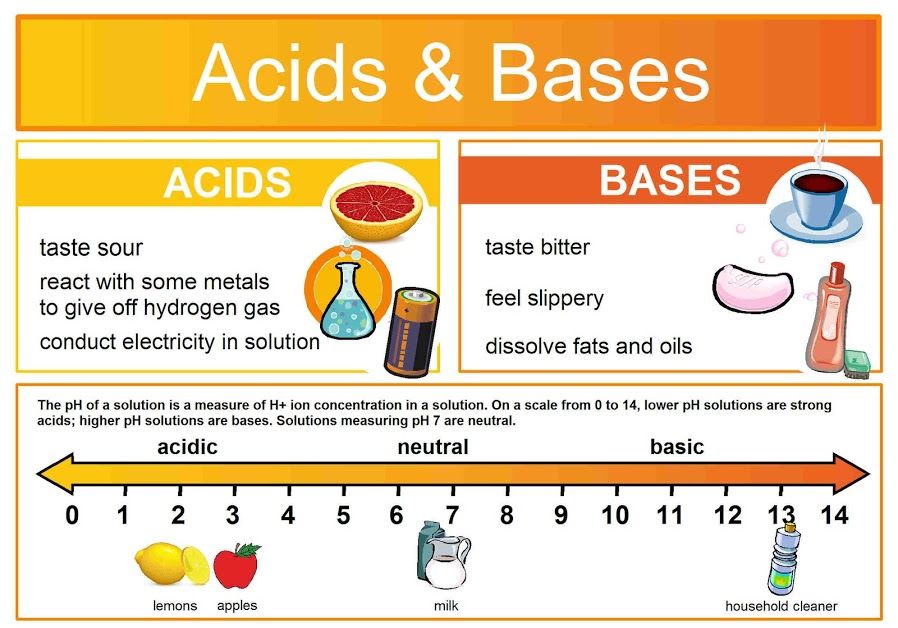
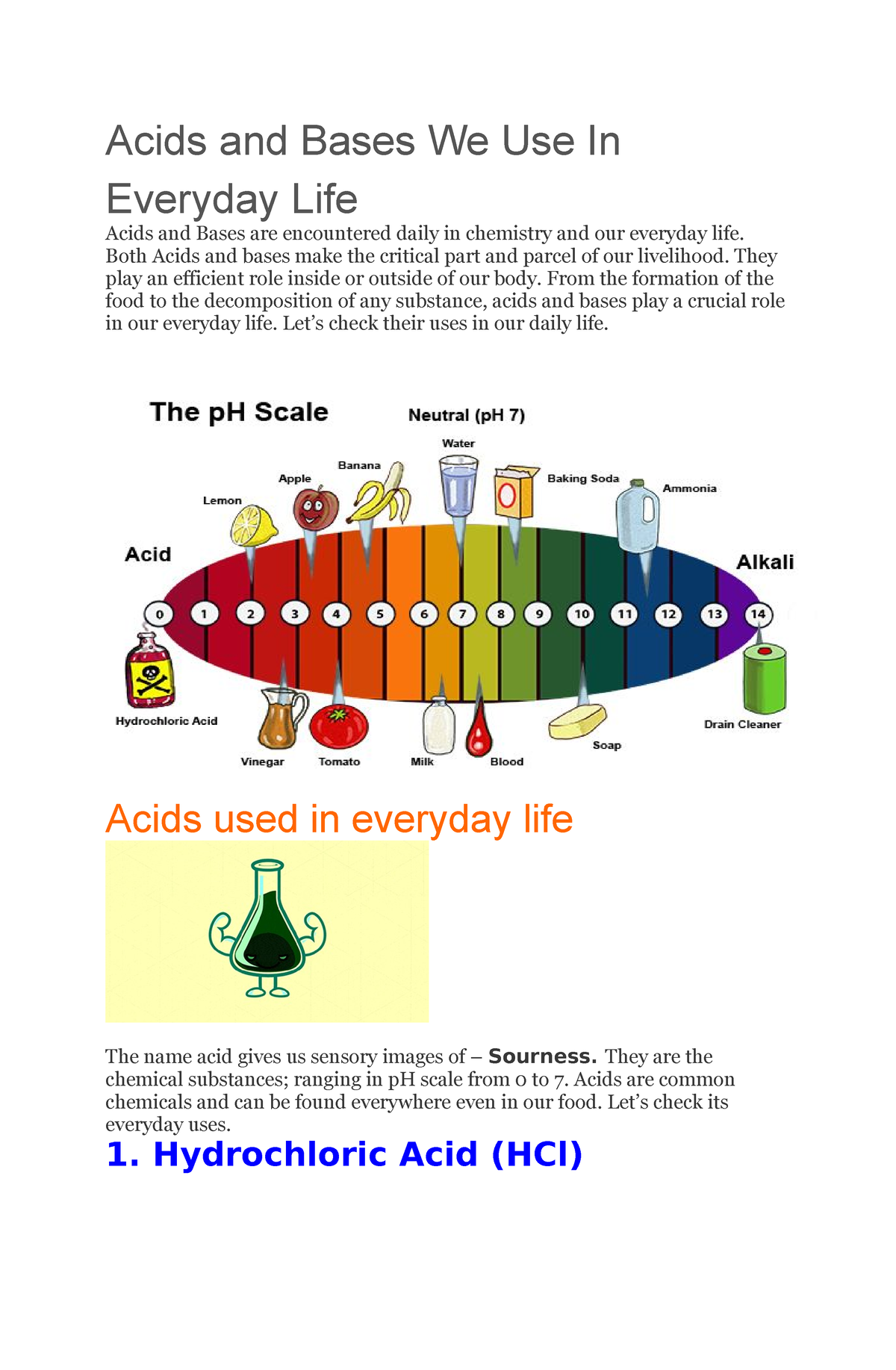
The pH scale is a logarithmic scale starting from 0 to 14, used to specific the acidity or basicity (alkalinity) of an aqueous resolution. A pH of seven is taken into account impartial, indicating an equal focus of hydrogen ions (H⁺) and hydroxide ions (OH⁻). Options with a pH lower than 7 are acidic, with the acidity rising because the pH decreases. Conversely, options with a pH better than 7 are primary (alkaline), with the basicity rising because the pH will increase. Every entire quantity change on the pH scale represents a tenfold change within the focus of hydrogen ions. For example, an answer with a pH of three is ten occasions extra acidic than an answer with a pH of 4, and 100 occasions extra acidic than an answer with a pH of 5.
The pH scale is not only a theoretical assemble; it has immense sensible significance. Many organic processes are extremely delicate to pH adjustments. For instance, the human physique maintains a fastidiously regulated pH inside a slender vary (barely alkaline) for optimum enzyme operate and general well being. Deviations from this vary can result in severe well being issues. Equally, many industrial processes require exact pH management to make sure environment friendly and protected operation.
Completely different Acid-Base Theories: Increasing the Understanding
A number of theories assist us perceive and classify acids and bases. The commonest are:
-
Arrhenius Principle: That is the best idea, defining acids as substances that produce hydrogen ions (H⁺) in aqueous resolution and bases as substances that produce hydroxide ions (OH⁻) in aqueous resolution. Whereas easy, this idea is proscribed because it solely applies to aqueous options and would not embody all substances that exhibit acidic or primary properties.
-
Brønsted-Lowry Principle: This idea supplies a broader definition. It defines an acid as a proton (H⁺) donor and a base as a proton acceptor. This idea extends the idea past aqueous options and encompasses a wider vary of drugs. For example, ammonia (NH₃) acts as a base by accepting a proton from water, regardless that it would not produce hydroxide ions immediately.
-
Lewis Principle: That is essentially the most complete idea, defining an acid as an electron-pair acceptor and a base as an electron-pair donor. This encompasses substances that do not essentially contain protons. For instance, boron trifluoride (BF₃) acts as a Lewis acid by accepting an electron pair from ammonia (a Lewis base).
Figuring out and Classifying Acids and Bases
Figuring out whether or not a substance is an acid or a base will be achieved by means of numerous strategies:
-
pH indicators: These are substances that change coloration relying on the pH of the answer. Litmus paper, a typical indicator, turns purple in acidic options and blue in primary options. Different indicators, like phenolphthalein and methyl orange, present extra exact pH ranges.
-
pH meters: These digital gadgets present a exact measurement of the pH of an answer. They’re extra correct than indicators and are extensively utilized in laboratories and industrial settings.
-
Titration: It is a quantitative technique used to find out the focus of an acid or base by reacting it with an answer of identified focus. The purpose at which the acid and base have fully neutralized one another is named the equivalence level, which will be decided utilizing indicators or pH meters.
Classifying Acids and Bases: Acids and bases will be additional categorised primarily based on their power and the variety of protons they’ll donate or settle for. Robust acids and bases fully dissociate in water, whereas weak acids and bases solely partially dissociate. Monoprotic acids donate one proton, diprotic acids donate two, and so forth. Equally, bases will be categorised primarily based on the variety of hydroxide ions they produce or the variety of protons they’ll settle for.
A Sensible Acid-Base Chart: Group and Interpretation
An acid-base chart is a useful device for organizing and evaluating the properties of various acids and bases. Such a chart may embody the next info:
| Substance | Chemical Formulation | Kind (Acid/Base) | Energy (Robust/Weak) | pH (approximate) | Makes use of | Hazards |
|---|---|---|---|---|---|---|
| Hydrochloric Acid | HCl | Acid | Robust | 0-1 | Industrial cleansing, abdomen acid | Corrosive |
| Sulfuric Acid | H₂SO₄ | Acid | Robust | 0-1 | Battery acid, fertilizer manufacturing | Corrosive, dehydrating |
| Nitric Acid | HNO₃ | Acid | Robust | 0-1 | Fertilizer manufacturing, explosives | Corrosive, oxidizing |
| Acetic Acid | CH₃COOH | Acid | Weak | 2-3 | Vinegar, meals preservative | Mildly irritating |
| Citric Acid | C₆H₈O₇ | Acid | Weak | 2-3 | Meals additive, cleansing agent | Typically protected |
| Sodium Hydroxide | NaOH | Base | Robust | 13-14 | Cleaning soap making, drain cleaner | Corrosive |
| Potassium Hydroxide | KOH | Base | Robust | 13-14 | Cleaning soap making, fertilizer manufacturing | Corrosive |
| Ammonia | NH₃ | Base | Weak | 11-12 | Cleansing agent, fertilizer manufacturing | Irritating, poisonous |
| Calcium Hydroxide | Ca(OH)₂ | Base | Weak | 12-13 | Mortar, plaster | Irritating |
It is a simplified instance. A complete chart may embody many extra substances, together with extra info resembling pKa (acid dissociation fixed) and pKb (base dissociation fixed) values, which offer a extra exact measure of acid and base power. The chart may be organized by power, sort (monoprotic, diprotic, and so forth.), or utility.
Purposes of Acid-Base Information
The understanding of acids and bases has far-reaching purposes throughout numerous fields:
-
Drugs: Sustaining correct pH stability within the physique is essential for well being. Antacids are used to neutralize abdomen acid, whereas intravenous fluids are fastidiously pH-balanced to keep away from hurt to tissues.
-
Business: Many industrial processes, such because the manufacturing of fertilizers, plastics, and prescription drugs, depend on exact pH management.
-
Agriculture: Soil pH impacts nutrient availability to vegetation. Farmers usually modify soil pH to optimize crop progress.
-
Environmental Science: Acid rain, brought on by the discharge of acidic pollution into the environment, has vital environmental penalties. Monitoring and mitigating acid rain requires an intensive understanding of acid-base chemistry.
-
Meals and Beverage Business: The pH of meals and drinks impacts their style, preservation, and security. Controlling pH is important for meals processing and preservation.
Conclusion
The examine of acids and bases is a cornerstone of chemistry, with far-reaching implications throughout many scientific disciplines and on a regular basis life. From understanding the intricacies of organic processes to creating new applied sciences and defending the surroundings, a strong grasp of acid-base chemistry is indispensable. The pH scale, together with numerous acid-base theories and sensible instruments like acid-base charts, present the framework for navigating this complicated but fascinating realm. By persevering with to refine our understanding of acids and bases, we are able to unlock new potentialities and handle essential challenges within the years to come back. The event and utility of latest indicators, extra exact measurement strategies, and superior computational fashions proceed to push the boundaries of our understanding and supply much more refined instruments for analyzing and using this elementary facet of chemistry.




Closure
Thus, we hope this text has offered useful insights into Decoding the Realm of Acids and Bases: A Complete Information to the pH Scale and Past. We respect your consideration to our article. See you in our subsequent article!