Understanding Metallic Precipitation pH Charts: A Complete Information
Associated Articles: Understanding Metallic Precipitation pH Charts: A Complete Information
Introduction
On this auspicious event, we’re delighted to delve into the intriguing subject associated to Understanding Metallic Precipitation pH Charts: A Complete Information. Let’s weave attention-grabbing data and supply recent views to the readers.
Desk of Content material
Understanding Metallic Precipitation pH Charts: A Complete Information
Metallic precipitation, the method the place dissolved steel ions transition from a soluble to an insoluble state, forming a strong precipitate, is essential in numerous fields, together with water therapy, environmental remediation, and industrial processes. Predicting and controlling this course of usually depends closely on understanding the connection between pH and steel solubility. This text delves into the intricacies of steel precipitation pH charts, explaining their development, interpretation, and functions, whereas highlighting limitations and issues.
The Chemistry Behind Metallic Precipitation and pH
Many metals exist in aqueous options as cations (positively charged ions). Their solubility, the power to stay dissolved, is considerably influenced by the answer’s pH. It is because pH impacts the focus of hydroxide ions (OH⁻). Hydroxide ions react with steel cations to kind steel hydroxides, lots of that are insoluble. The overall response may be represented as:
Mⁿ⁺(aq) + nOH⁻(aq) ⇌ M(OH)ₙ(s)
the place:
- Mⁿ⁺ represents the steel cation with a cost of n+
- OH⁻ represents the hydroxide ion
- M(OH)ₙ represents the steel hydroxide precipitate
The equilibrium fixed for this response, often called the solubility product fixed (Ksp), determines the solubility of the steel hydroxide at a given temperature. A decrease Ksp signifies decrease solubility, which means the steel is extra more likely to precipitate. The Ksp worth is temperature-dependent, and charts sometimes assume a normal temperature (e.g., 25°C).
Establishing a Metallic Precipitation pH Chart
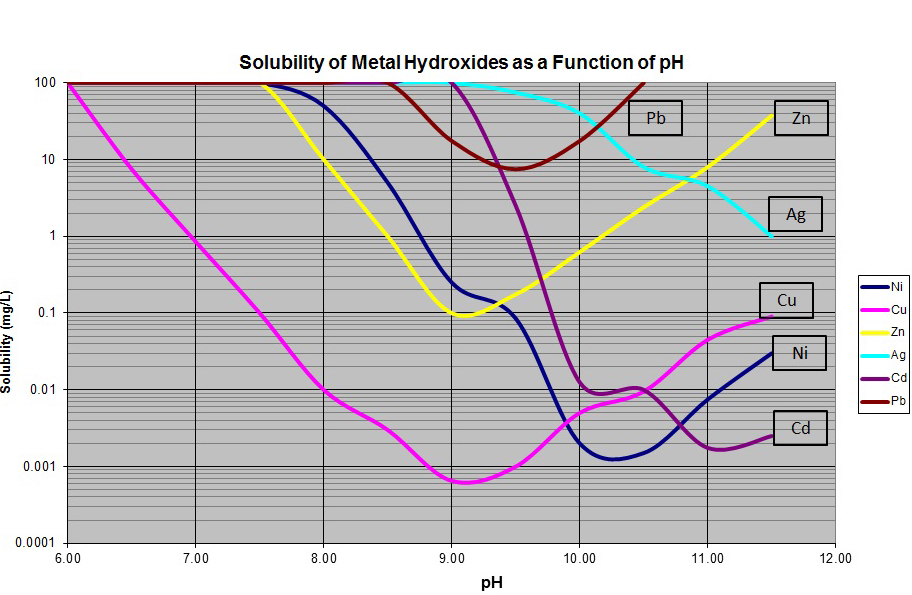
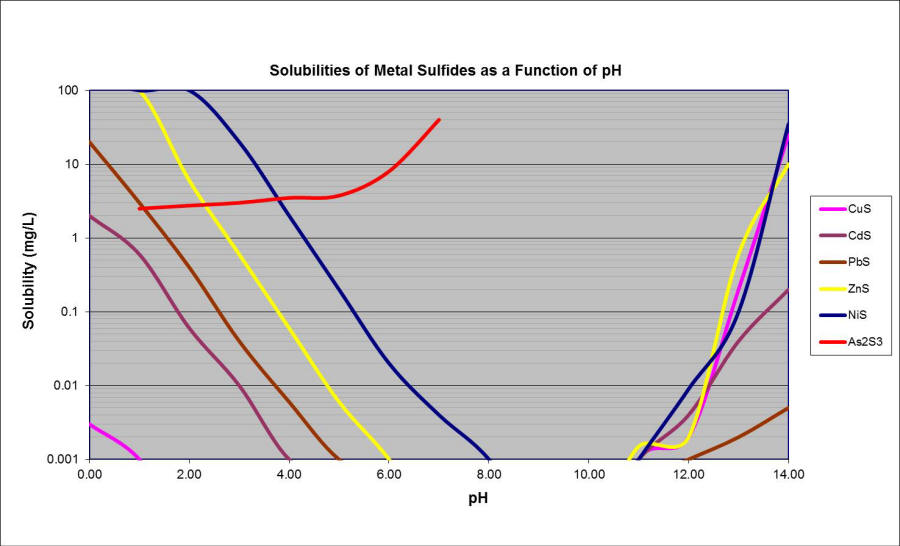
Metallic precipitation pH charts are graphical representations of the connection between pH and the focus of dissolved steel ions remaining in answer after precipitation. They’re sometimes constructed utilizing thermodynamic knowledge, particularly the Ksp values of the steel hydroxides and the related equilibrium reactions. The chart often shows the focus of the dissolved steel ion (usually expressed in mg/L or ppm) on the y-axis and the pH on the x-axis.
The development course of includes:
-
Figuring out related equilibrium reactions: This consists of contemplating the formation of assorted steel hydroxide species (e.g., M(OH)⁺, M(OH)₂⁰, and many others.) and their respective equilibrium constants. The complexity will increase with the steel’s oxidation state and the presence of different ligands within the answer.
-
Calculating solubility at totally different pH values: Utilizing the Ksp and equilibrium constants, the focus of the dissolved steel ion is calculated for a variety of pH values. This includes fixing a collection of simultaneous equations, usually requiring computational instruments.
-
Plotting the information: The calculated knowledge factors (pH vs. dissolved steel focus) are plotted on a graph, creating the precipitation pH chart. The chart usually consists of a number of curves, every representing a special steel or a special steel species.
-
Figuring out the precipitation area: The area beneath the curve represents the pH vary the place the steel is predominantly precipitated. Above the curve, the steel stays largely dissolved.
Decoding a Metallic Precipitation pH Chart
A typical steel precipitation pH chart gives precious data:
-
pH required for precipitation: The chart straight exhibits the minimal pH required to attain a particular degree of steel removing from answer. For instance, it’d point out the pH wanted to scale back the focus of a selected steel to beneath a regulatory restrict.
-
Impact of pH on steel solubility: The slope of the curve illustrates the sensitivity of steel solubility to pH adjustments. A steep slope signifies a robust dependence on pH, whereas a gradual slope suggests much less sensitivity.
-
Comparability of steel habits: A number of curves permit for a direct comparability of the precipitation habits of various metals underneath the identical circumstances. This helps in understanding the relative ease of eradicating totally different metals from an answer by way of pH adjustment.
-
Prediction of precipitation: The chart can predict whether or not a steel will precipitate underneath a particular set of circumstances (pH and preliminary steel focus).
Functions of Metallic Precipitation pH Charts
Metallic precipitation pH charts discover widespread functions in numerous fields:
-
Water Remedy: In wastewater therapy crops, pH adjustment is a typical approach for eradicating heavy metals. Charts assist optimize the pH for environment friendly steel removing whereas minimizing the usage of chemical substances.
-
Environmental Remediation: Contaminated soil and groundwater usually include elevated ranges of heavy metals. pH adjustment, guided by precipitation charts, can facilitate the removing of those metals throughout remediation processes.
-
Hydrometallurgy: Within the extraction of metals from ores, controlling pH is essential for selective precipitation of desired metals and separation from undesirable impurities.
-
Chemical Evaluation: Precipitation is usually employed in analytical chemistry for separating and quantifying metals. pH charts assist in optimizing precipitation circumstances for correct evaluation.
Limitations and Concerns
Whereas steel precipitation pH charts are highly effective instruments, they’ve limitations:
-
Simplified fashions: The charts usually simplify complicated chemical methods by neglecting the affect of different ions, complexation reactions, and temperature variations. Actual-world methods are sometimes extra intricate.
-
Thermodynamic equilibrium: The charts are based mostly on thermodynamic equilibrium, which can not at all times be achieved in follow, particularly in dynamic methods. Kinetic components can affect the precise precipitation price.
-
Temperature dependence: Ksp values are temperature-dependent. Charts constructed for a particular temperature will not be correct at different temperatures.
-
Interference from different ions: The presence of different ions in answer can considerably have an effect on steel solubility and precipitation habits. The charts could not precisely predict habits in complicated mixtures.
-
Strong part characterization: The chart would not present details about the character of the precipitated strong. The precipitate may be amorphous or crystalline, and its properties can have an effect on its dealing with and disposal.
Conclusion
Metallic precipitation pH charts are precious instruments for understanding and predicting the habits of metals in aqueous options. They supply a handy graphical illustration of the connection between pH and steel solubility, enabling optimization of processes in numerous fields. Nonetheless, it is essential to acknowledge the constraints of those charts and take into account the complexities of real-world methods. Utilizing these charts successfully requires an intensive understanding of the underlying chemistry and a cautious consideration of the potential influencing components. Combining chart knowledge with experimental verification and extra subtle modeling strategies ensures a extra correct and dependable prediction of steel precipitation habits.




![pH for metal hydroxide precipitation [59] Download Scientific Diagram](https://www.researchgate.net/profile/Abdulwasiu-Salaudeen-Olawale-2/publication/344986688/figure/tbl5/AS:952210330492931@1604036042892/pH-for-metal-hydroxide-precipitation-59.png)

Closure
Thus, we hope this text has supplied precious insights into Understanding Metallic Precipitation pH Charts: A Complete Information. We thanks for taking the time to learn this text. See you in our subsequent article!