Understanding the pH Chart: A Complete Information to Acidity and Alkalinity
Associated Articles: Understanding the pH Chart: A Complete Information to Acidity and Alkalinity
Introduction
On this auspicious event, we’re delighted to delve into the intriguing subject associated to Understanding the pH Chart: A Complete Information to Acidity and Alkalinity. Let’s weave attention-grabbing data and supply contemporary views to the readers.
Desk of Content material
Understanding the pH Chart: A Complete Information to Acidity and Alkalinity

The pH chart, a easy but highly effective device, supplies a visible illustration of the acidity or alkalinity of an answer. Understanding its implications is essential throughout varied fields, from chemistry and biology to agriculture and environmental science. This text delves deep into the intricacies of the pH chart, exploring its scale, its significance, and its functions in numerous contexts.
The pH Scale: A Measure of Hydrogen Ion Focus
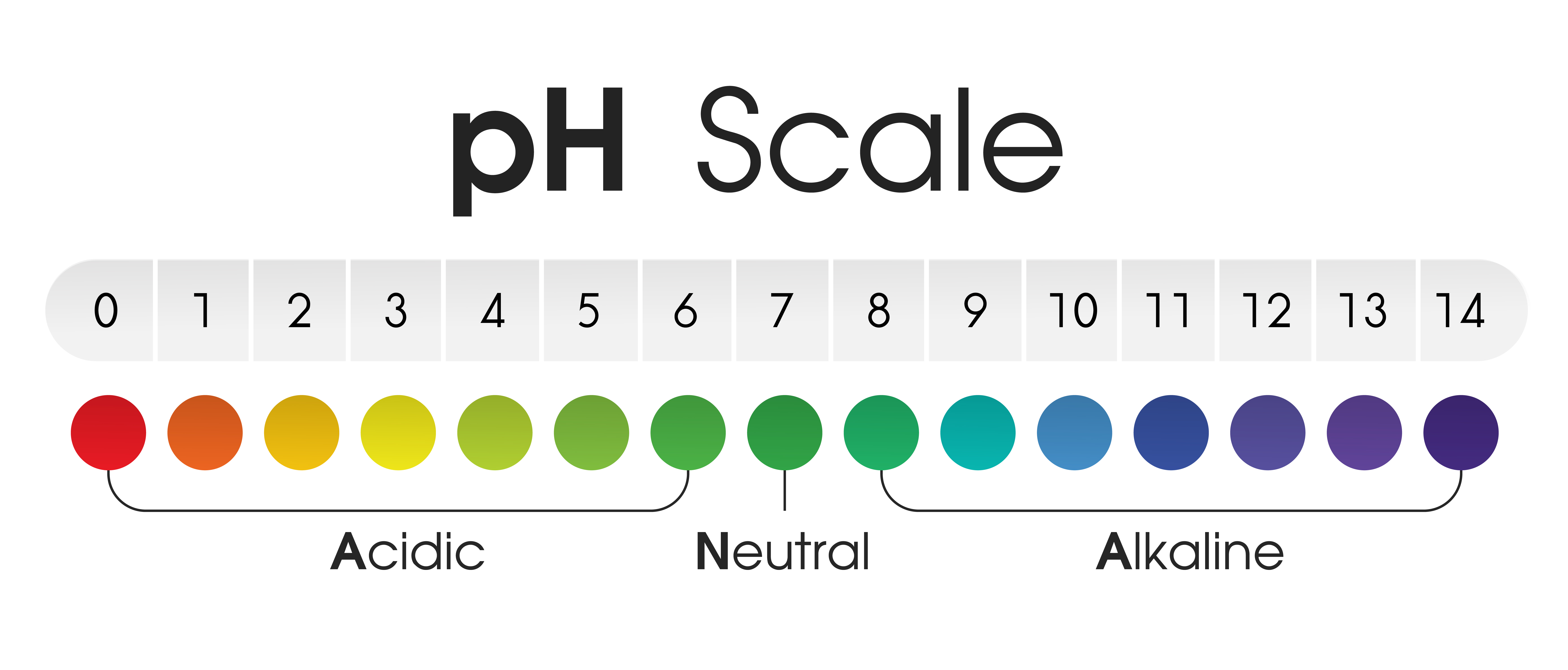
The pH scale is a logarithmic scale starting from 0 to 14, indicating the focus of hydrogen ions (H⁺) in an answer. A decrease pH worth signifies the next focus of H⁺ ions, indicating a extra acidic resolution. Conversely, the next pH worth signifies a decrease focus of H⁺ ions and a extra alkaline (or primary) resolution. The midpoint of the size, pH 7, represents neutrality, the place the focus of H⁺ ions equals the focus of hydroxide ions (OH⁻).
The logarithmic nature of the size is essential. Every complete quantity change on the pH scale represents a tenfold change within the focus of hydrogen ions. As an example, an answer with a pH of three is ten instances extra acidic than an answer with a pH of 4, and 100 instances extra acidic than an answer with a pH of 5. This exponential relationship highlights the numerous impression even small modifications in pH can have.
Decoding the pH Chart:
A typical pH chart visually represents the pH scale, usually with color-coded sections to simply establish the acidity or alkalinity of a substance. These colours usually correspond to the pH ranges generally encountered in numerous functions. For instance:
-
pH 0-3 (Extremely Acidic): This vary represents options with extraordinarily excessive concentrations of hydrogen ions. Sturdy acids like hydrochloric acid (HCl) and sulfuric acid (H₂SO₄) fall inside this vary. These options are corrosive and may trigger important injury to residing tissues and supplies.
-
pH 4-6 (Acidic): This vary consists of options which might be nonetheless acidic however much less so than the extremely acidic vary. Examples embody vinegar (acetic acid) and lemon juice (citric acid).
-
pH 7 (Impartial): Pure water at 25°C has a pH of seven. This represents a balanced state the place the focus of hydrogen and hydroxide ions are equal.
-
pH 8-10 (Alkaline): This vary signifies options with a decrease focus of hydrogen ions and the next focus of hydroxide ions. Examples embody baking soda (sodium bicarbonate) and seawater.
-
pH 11-14 (Extremely Alkaline): This vary represents options with extraordinarily low concentrations of hydrogen ions and excessive concentrations of hydroxide ions. Sturdy bases like sodium hydroxide (NaOH) and potassium hydroxide (KOH) fall inside this vary. These options are additionally corrosive and will be dangerous.
The Significance of pH in Totally different Fields:
The pH chart performs a vital function in all kinds of fields:
1. Chemistry: Understanding pH is prime to many chemical reactions and processes. The pH of an answer can have an effect on the speed and course of a response, the solubility of gear, and the soundness of chemical compounds. Titration, a typical laboratory approach, depends closely on pH measurements to find out the focus of unknown options.
2. Biology: pH performs a significant function in organic programs. The pH of blood, as an illustration, is tightly regulated inside a slender vary (round 7.4) to take care of optimum physiological operate. Deviations from this vary can result in critical well being penalties. Enzymes, the organic catalysts that drive many life processes, are extremely delicate to pH modifications, with their exercise usually being optimum inside a selected pH vary. Equally, the expansion and survival of microorganisms are considerably influenced by the pH of their surroundings.
3. Agriculture: Soil pH is a essential issue influencing plant development and nutrient availability. Totally different vegetation thrive in numerous pH ranges. Farmers usually monitor and alter soil pH to optimize crop yields. The pH of irrigation water additionally performs a major function, as excessive acidity or alkalinity can injury plant roots and have an effect on nutrient uptake.
4. Environmental Science: pH is a key indicator of water high quality. Acid rain, attributable to atmospheric pollution, lowers the pH of lakes and rivers, harming aquatic life. Monitoring pH ranges in water our bodies is essential for assessing environmental well being and defending ecosystems. Moreover, pH performs a major function in wastewater remedy processes, the place it is rigorously managed to optimize the effectiveness of assorted remedy steps.
5. Meals and Beverage Business: pH management is crucial in meals processing and preservation. The pH of meals merchandise influences their style, texture, and shelf life. Many meals preservation methods, reminiscent of pickling and canning, depend on adjusting the pH to inhibit microbial development. The brewing and winemaking industries additionally rigorously monitor and management pH all through the fermentation course of to make sure the standard of the ultimate product.
6. Drugs: pH performs a vital function in varied medical functions. The pH of bodily fluids, reminiscent of blood and urine, supplies invaluable diagnostic data. Many medicines are formulated to have particular pH values for optimum absorption and efficacy. Moreover, pH is a crucial consider wound therapeutic and the remedy of sure pores and skin circumstances.
Measuring pH:
A number of strategies can be found for measuring pH:
-
pH Indicators: These are substances that change colour relying on the pH of the answer. Litmus paper, a typical pH indicator, turns crimson in acidic options and blue in alkaline options. Extra refined indicators present a broader vary of colour modifications, permitting for extra exact pH willpower.
-
pH Meters: These digital gadgets present a extra correct and exact measurement of pH. They use a pH-sensitive electrode to measure the potential distinction between the electrode and a reference electrode, which is straight associated to the pH of the answer. pH meters are broadly utilized in laboratories and industrial settings.
Conclusion:
The pH chart serves as a basic device for understanding and managing acidity and alkalinity throughout quite a few disciplines. Its logarithmic scale highlights the numerous impression of even refined modifications in hydrogen ion focus. From the microscopic world of cells to the huge expanse of ecosystems, the pH of an answer exerts a profound affect on chemical reactions, organic processes, and environmental circumstances. By understanding and using the knowledge offered by the pH chart, we are able to successfully monitor, management, and optimize processes in varied fields, contributing to developments in science, know-how, and environmental stewardship. Steady monitoring and cautious administration of pH are essential for making certain optimum outcomes in various functions, underscoring the importance of this seemingly easy but highly effective device.






Closure
Thus, we hope this text has offered invaluable insights into Understanding the pH Chart: A Complete Information to Acidity and Alkalinity. We thanks for taking the time to learn this text. See you in our subsequent article!