Unveiling the Valence Chart: A Deep Dive into Elemental Reactivity
Associated Articles: Unveiling the Valence Chart: A Deep Dive into Elemental Reactivity
Introduction
With nice pleasure, we’ll discover the intriguing subject associated to Unveiling the Valence Chart: A Deep Dive into Elemental Reactivity. Let’s weave attention-grabbing info and provide contemporary views to the readers.
Desk of Content material
Unveiling the Valence Chart: A Deep Dive into Elemental Reactivity
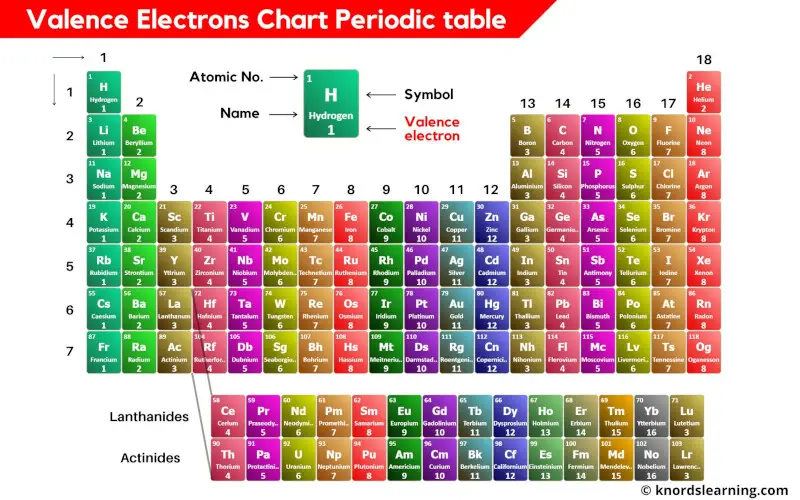
The periodic desk, a cornerstone of chemistry, organizes components based mostly on their atomic construction and recurring properties. Understanding a component’s conduct, significantly its reactivity, hinges on comprehending its valence electrons – the outermost electrons concerned in chemical bonding. A valence chart, although not a formally standardized doc just like the periodic desk itself, is a visible illustration of those essential valence electrons for every ingredient. This text delves into the idea of valence, explores the development and interpretation of a valence chart, and discusses its implications for predicting chemical conduct.
Understanding Valence Electrons and Valence:
Valence electrons are the important thing gamers in chemical reactions. They decide a component’s bonding capability and the forms of bonds it may possibly kind (ionic, covalent, metallic). The variety of valence electrons an atom possesses is instantly associated to its place throughout the periodic desk, particularly its group (vertical column). Components throughout the similar group share the identical variety of valence electrons, resulting in comparable chemical properties.
-
Group 1 (Alkali Metals): These components have one valence electron, making them extremely reactive. They readily lose this electron to realize a steady noble fuel configuration.
-
Group 2 (Alkaline Earth Metals): Possessing two valence electrons, these metals are additionally reactive, although much less so than alkali metals. They have an inclination to lose each electrons to realize stability.
-
Teams 13-17: The variety of valence electrons will increase throughout these teams, resulting in a extra complicated bonding conduct. Components in these teams can acquire, lose, or share electrons to realize stability.
-
Group 18 (Noble Gases): These components have a full valence shell (eight electrons, apart from helium with two), making them exceptionally unreactive. Their steady electron configuration is the driving drive behind the reactivity of different components.
Developing a Valence Chart:
A valence chart may be constructed in numerous methods, relying on the extent of element required. A easy chart may record the ingredient’s image and its variety of valence electrons. A extra complete chart might embrace:
- Aspect Image: The usual chemical image (e.g., H, O, Na, Cl).
- Atomic Quantity: The variety of protons within the nucleus (and subsequently, electrons in a impartial atom).
- Electron Configuration: The association of electrons in shells and subshells (e.g., 1s²2s²2p⁶3s¹ for sodium).
- Valence Electrons: The variety of electrons within the outermost shell.
- Widespread Oxidation States: The cost an atom acquires when it loses or positive aspects electrons in a chemical response. This may differ relying on the precise compound.
- Typical Bonding Habits: A short description of the kind of bonds the ingredient usually kinds (ionic, covalent, metallic).
Deciphering a Valence Chart:
A well-constructed valence chart permits for fast predictions about a component’s chemical conduct. For instance:
-
Predicting Reactivity: Components with one or two valence electrons (alkali and alkaline earth metals) are extremely reactive as a result of their tendency to lose electrons and obtain a steady octet. Components with seven valence electrons (halogens) are additionally extremely reactive, as they readily acquire one electron to finish their octet.
-
Predicting Bond Sort: Components with considerably totally different electronegativities (a measure of an atom’s capability to draw electrons) are prone to kind ionic bonds (switch of electrons). Components with comparable electronegativities are inclined to kind covalent bonds (sharing of electrons). Metals usually kind metallic bonds, characterised by a sea of delocalized electrons.
-
Predicting Compound Formation: By understanding the valence electrons of the constituent components, one can predict the possible formulation of a compound. As an illustration, sodium (Na, one valence electron) and chlorine (Cl, seven valence electrons) will react to kind NaCl (sodium chloride), the place sodium loses one electron and chlorine positive aspects one, leading to steady ions with full octets.
Limitations of a Valence Chart:
Whereas a valence chart is a useful software, it has limitations:
-
Transition Metals: Transition metals have extra complicated valence electron configurations, and their oxidation states can differ considerably relying on the response circumstances. A easy valence chart may not precisely replicate this complexity.
-
Exceptions to the Octet Rule: Some components, significantly these in intervals past the third, can kind compounds that violate the octet rule (having kind of than eight valence electrons).
-
Dynamic Nature of Bonding: Bonding just isn’t at all times simple. Resonance constructions and polar covalent bonds add layers of complexity not totally captured by a easy valence chart.
Examples and Purposes:
Let’s take into account just a few examples as an instance the utility of a valence chart:
-
Predicting the formulation of magnesium oxide (MgO): Magnesium (Mg) has two valence electrons, and oxygen (O) has six. Magnesium will lose two electrons to develop into Mg²⁺, and oxygen will acquire two electrons to develop into O²⁻. The ensuing compound has a 1:1 ratio of Mg²⁺ and O²⁻ ions, resulting in the formulation MgO.
-
Understanding the reactivity of fluorine: Fluorine (F) has seven valence electrons and is extremely reactive as a result of it readily positive aspects one electron to realize a steady octet. This explains its sturdy oxidizing energy.
-
Explaining the inertness of neon: Neon (Ne) has eight valence electrons (a full octet), making it extraordinarily unreactive. It doesn’t readily lose or acquire electrons.
Past the Fundamental Valence Chart:
Extra superior valence charts can incorporate ideas like:
- Formal Cost: A technique for assigning prices to atoms in molecules, serving to to find out essentially the most steady Lewis construction.
- Electronegativity Variations: Illustrating the polarity of covalent bonds.
- Molecular Orbital Idea: A extra subtle mannequin of bonding that considers the interactions of atomic orbitals to kind molecular orbitals.
Conclusion:
A valence chart, whereas a simplified illustration, gives a strong software for understanding and predicting the chemical conduct of components. By visualizing the variety of valence electrons and their implications for bonding, we acquire insights into reactivity, bond varieties, and compound formation. Whereas limitations exist, significantly with transition metals and exceptions to the octet rule, a well-interpreted valence chart stays a vital useful resource for college students and chemists alike, providing a foundational understanding of the basic ideas governing chemical reactions. Additional exploration of superior bonding theories gives a extra full image, however the primary valence chart stays a useful start line within the journey of understanding chemical reactivity.






Closure
Thus, we hope this text has supplied useful insights into Unveiling the Valence Chart: A Deep Dive into Elemental Reactivity. We thanks for taking the time to learn this text. See you in our subsequent article!